Nucleophilic addition to N-alkoxyamides
Abstract
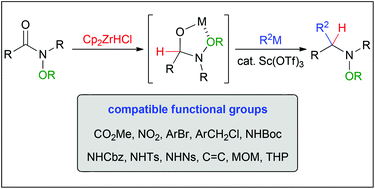
An amide group is one of the most abundant functional groups in organic synthesis. However, nucleophilic addition to amide carbonyls has received less attention than their construction due to their high stability. In this Perspective, we describe our recent progress with N-alkoxyamides. Incorporation of an N-alkoxy group as a reactivity control element into the nitrogen atom of an amide successfully overcomes issues inherent to the nucleophilic addition. The reaction can introduce two different nucleophiles in a one-pot process, giving a variety of substituted amines. When the Schwartz reagent was used in the first reduction step, high chemoselectivity was observed in the presence of sensitive functional groups such as esters, which resulted in the concise total synthesis of (±)-gephyrotoxin.

 Please wait while we load your content...
Please wait while we load your content...