Synthesis and gene transfection activity of cyclen-based cationic lipids with asymmetric acyl-cholesteryl hydrophobic tails
Abstract
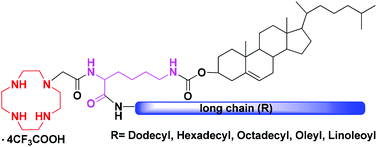
A series of novel 1,4,7,10-tetraazacyclododecane (cyclen)-based cationic lipids with asymmetric double hydrophobic tails (cholesteryl and long aliphatic chains) were designed and synthesized. Lysine was chosen as a linking moiety in the molecular backbone. The liposomes formed from 8 and dioleoylphosphatidylethanolamine (DOPE) could bind and condense plasmid DNA into nanoparticles under a low N/P ratio. These nano-scaled lipoplexes have low cytotoxicity, and might efficiently transfect A549 cells. In vitro transfection results revealed that all cationic lipids showed a comparable or better transfection efficiency (TE) than commercially available Lipofectamine 2000. The length and saturation degree of the aliphatic chain would affect their gene transfection performance, and the linoleic acid-containing 8e could give the best TE.

 Please wait while we load your content...
Please wait while we load your content...