Asymmetric synthesis of chloroisothreonine derivatives via syn-stereoselective Mannich-type additions across N-sulfinyl-α-chloroimines†
Abstract
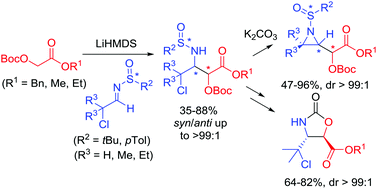
Mannich-type reactions of O-Boc glycolic esters across chiral N-sulfinyl-α-chloroaldimines resulted in the efficient and syn-stereoselective synthesis of new γ-chloro-α-hydroxy-β-amino esters (dr > 99 : 1). The α-coordinating ability of the chlorine atom was of great importance for the diastereoselectivity of the Mannich-type reaction and overruled the chelation of the sulfinyl oxygen with the lithium ion of the incoming E-enolate in the transition state model. These novel chloroisothreonine derivatives proved to be excellent building blocks in asymmetric synthesis of novel syn-β,γ-aziridino-α-hydroxy esters and biologically relevant trans-oxazolidinone carboxylic esters.

 Please wait while we load your content...
Please wait while we load your content...