The diene-transmissive hetero-Diels–Alder reaction of 2-vinyl α,β-unsaturated aldimines: stereoselective synthesis of hexahydroquinazolin-2-ones†
Abstract
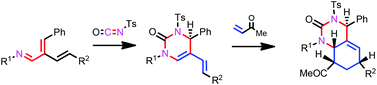
Stereoselective synthesis of hexahydroquinazolin-2(1H)-ones has been achieved through the application of the diene-transmissive hetero-Diels–Alder methodology to 2-vinyl-1-aza-1,3-butadienes. The cross-conjugated 1-azatriene underwent an initial hetero-Diels–Alder reaction on the 1-aza-1,3-butadiene system with tosyl isocyanate to afford the [4 + 2] mono-cycloadduct pyrimidinone. The second Diels–Alder reaction on the electron-rich 1-amino-1,3-diene unit of the mono-cycloadduct with dienophiles provided hexahydroquinazolin-2(1H)-ones with high stereoselectivity.

 Please wait while we load your content...
Please wait while we load your content...