Structure–property relationships in a series of diglycerol tetraether model lipids and their lyotropic assemblies: the effect of branching topology and chirality†
Abstract
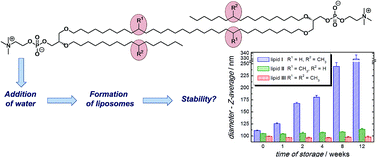
Three novel diglycerol tetraether lipids with one membrane-spanning chain have been synthesized. These lipids contain only two or four racemic methyl branches at selected positions of the hydrophobic chains in contrast to natural lipids from archaebacterial membranes with an isoprenoid substitution pattern. The insertion of the methyl moieties was realized starting from either (RS)-citronellyl bromide or the inexpensive methyl malonic acid ethyl ester. For chain elongation the Cu-catalysed Grignard coupling reaction was used. The preparation of diglycerol tetraethers was either performed by condensing suitable blocked monoglycerol diethers by Grubbs metathesis or by reaction of the transmembrane C32-chain with blocked glycerols followed by further alkylation steps. Finally, we could show that the resulting lipids can form closed lipid vesicles comparable to the optically pure counterparts. Therefore, these much simpler lipids compared to the natural lipids from archaebacterial membranes are also suitable for preparation of stable tailored liposomes.

 Please wait while we load your content...
Please wait while we load your content...