Carboxy-directed asymmetric hydrogenation of α-alkyl-α-aryl terminal olefins: highly enantioselective and chemoselective access to a chiral benzylmethyl center†
Abstract
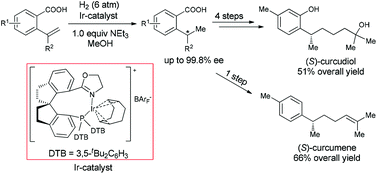
A carboxy-directed asymmetric hydrogenation of α-alkyl-α-aryl terminal olefins was developed by using a chiral spiro iridium catalyst, providing a highly efficient approach to the compounds with a chiral benzylmethyl center. The carboxy-directed hydrogenation prohibited the isomerization of the terminal olefins, and realized the chemoselective hydrogenation of various dienes. The concise enantioselective syntheses of (S)-curcudiol and (S)-curcumene were achieved by using this catalytic asymmetric hydrogenation as a key step.

 Please wait while we load your content...
Please wait while we load your content...