Palladium catalyzed acetoxylation of benzylic C–H bonds using a bidentate picolinamide directing group†
Abstract
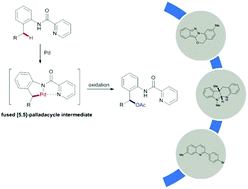
A general palladium catalyzed acetoxylation of benzylic C–H bonds has been developed. Picolinamides serve as an excellent directing group for the C–H activation of benzylic methyls. A wide range of 2-amino benzyl alcohol analogues were synthesized in good yields. The products demonstrated broad synthetic utilities toward various benzo-fused heterocycles. Mechanistic studies revealed the key rate-limiting C–H insertion step, which could be affected by the substitution pattern of the parent arene.

 Please wait while we load your content...
Please wait while we load your content...