Biocatalyst mediated regio- and stereo-selective hydroxylation and epoxidation of (Z)-α-santalol†
Abstract
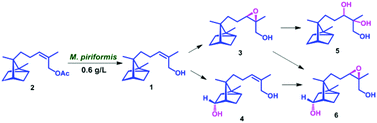
Biocatalyst mediated regio- and stereo-selective hydroxylation and epoxidation on (Z)-α-santalol were achieved for the first time, using a fungal strain Mucor piriformis. Four novel metabolites were characterized as 10,11-cis-β-epoxy-α-santalol, 5α-hydroxy-(Z)-α-santalol, 10,11-dihydroxy-α-santalol and 5α-hydroxy-10,11-cis-β-epoxy-α-santalol. Using Amano PS lipase from Burkholderia cepacia, α- and β-isomers of 10,11-cis-epoxy-α-santalol were resolved efficiently.

 Please wait while we load your content...
Please wait while we load your content...