Ylide mediated carbonyl homologations for the preparation of isatin derivatives†
Abstract
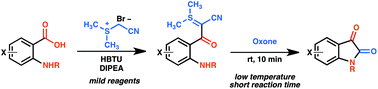
An exceptionally mild method for the preparation of isatin derivatives has been developed using a sulfur ylide mediated carbonyl homologation sequence starting from anthranilic acid precursors. This method proceeds at ambient temperature via a sulfur ylide intermediate without the need for protection of the amine or chromatographic isolation of the intermediate ylide. Gentle oxidation of the sulfur ylides provides isatin derivatives with N–H, N-alkyl, N-aryl substitution, electron-rich and electron-poor aromatic rings, and heterocyclic aromatic systems. We anticipate that this method will greatly expand the accessibility of complex isatin derivatives.

 Please wait while we load your content...
Please wait while we load your content...