Unraveling polar Diels–Alder reactions with conceptual DFT analysis and the distortion/interaction model†
Abstract
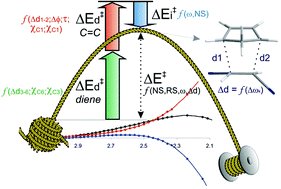
The reaction energetics of 280 polar Diels–Alder (DA) reactions between 70 dienophiles and 4 dienes have been studied in detail using the B3LYP/6-31G* level of theory, combining conceptual density functional theory (DFT) analysis and the distortion/interaction model. The barrier heights are governed by a fine balance between the energy required to distort the reactants from their initial to their transition state geometries (ΔE‡d) and the binding energy between the deformed reactants in the TS (ΔE‡i). The ΔE‡i values strongly correlate with the electrophilicity index, ω, which measures the stabilization energy when the system acquires an additional electronic charge from the environment, whereas the ΔE‡d was found to depend mainly on the nature of the diene, structural parameters of the dienophile (degree of substitution and ring size) and the asynchronicity of the TS. A detailed analysis to account for the geometrical parameters of the strained diene and dienophile moieties that influence the energy strain of the distorted fragments is also reported.

 Please wait while we load your content...
Please wait while we load your content...