Synthesis of new bioorganometallic Ir- and Rh-complexes having β-lactam containing ligands†
Abstract
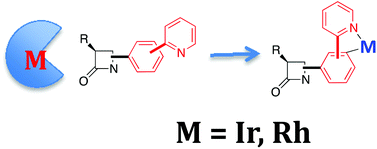
The synthesis (and full spectroscopic and crystallographic characterization) of new classes of bioorganometallic Ir- and Rh-complexes having β-lactam containing ligands has been achieved in three steps starting from simple precursors. The procedure for preparing these bioorganometallic compounds uses β-lactams having a phenylpyridyl moiety attached to the C4, N1 or C4 and N1 positions simultaneously, and a directed C–H metal-insertion, in the presence of (MCp*Cl2)2 (M = Ir, Rh). Enantiomerically pure 2-azetidinones can be transformed into diastereomeric (at the metal) mixtures of enantiopure metalla-2-azetidinones. Bimetallic 2-azetidinones are also accessible by this approach. The insertion of electron-poor alkynes into the M–C bond of the bioorganometallic complex occurs regioselectively and in excellent yields. Overall, the sequence imine-β-lactam–metalla-β-lactam is a versatile and efficient full methodology to prepare and functionalize unprecedented, novel Ir- and Rh-complexes having β-lactam containing ligands.

 Please wait while we load your content...
Please wait while we load your content...