Halide ion-mediated growth of single crystalline Fe nanoparticles†
Abstract
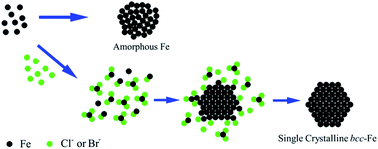
We report a facile halide ion (Cl− or Br−) mediated synthesis of Fe nanoparticles (NPs) by thermal decomposition of Fe(CO)5. The NP structure is controlled to be either amorphous (in the absence of halide ions) or single crystalline bcc (in the presence of halide ions). Through systematic investigation on the synthetic conditions, we have confirmed that the formation of bcc-Fe NPs is facilitated by the strong interactions between halide ions and Fe, which favor thermodynamic growth of Fe over the existing Fe NPs. Compared with the amorphous Fe NPs, the bcc-Fe NPs exhibit much enhanced magnetization values and chemical stability. This halide ion mediated growth may become a general strategy to control the growth of metallic NPs, especially first-row transition metal NPs, in a thermodynamically more stable way, producing single crystalline NPs with much controlled physical and chemical properties for magnetic and catalytic applications.

 Please wait while we load your content...
Please wait while we load your content...