Unusual effects of solvent polarity on capacitance for organic electrolytes in a nanoporous electrode
Abstract
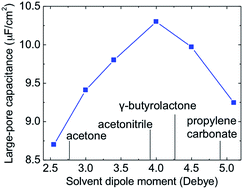
The interplay between ions and solvent molecules inside the nanoporous electrodes of a supercapacitor has not been well understood but could be a fertile ground for new insights into the device’s performance. By tuning the dipole moment of the solvent in an organic electrolyte, we find, from classical density functional theory calculations, pronounced oscillation of capacitance with the pore size for a moderately to weakly polar solvent. A quantitative analysis of the electric-double-layer (EDL) structure indicates that the capacitance oscillation shares a similar physical origin to that of an ionic liquid electrolyte: the oscillatory behavior arises from the formation of alternating layers of counterions and coions near strongly charged surfaces. More interestingly, we find that in the large-pore region, the capacitance versus the pore size has a volcano-shaped trend; in other words, there exists a solvent dipole moment that yields a maximal capacitance. These theoretical predictions can be validated with future experiments and highlight the great potential in tuning the organic solvent to achieve optimal performance of EDL capacitors.

 Please wait while we load your content...
Please wait while we load your content...