Chemo- and site-selective derivatizations of natural products enabling biological studies
Abstract
Covering: 2006 to July 2013
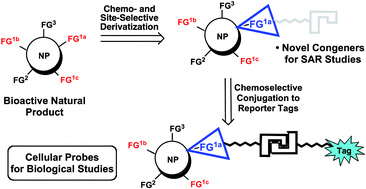
Bioactive natural products and derivatives remain an enduring starting point for the discovery of new cellular targets for disease intervention and lead compounds for the development of new therapeutic agents. The former goal is accomplished through the synthesis of bioactive cellular probes from natural products, enabling insights into the mechanism of action of these natural products by classical affinity chromatography or more recent proteome profiling methods. However, the direct and selective modification of native natural products for these purposes remains a challenge due to the structural complexity and the wide functional group diversity found in these natural substances. The lack of selective synthetic methods available to directly manipulate unprotected complex small molecules, in particular to perform structure–activity relationship studies and prepare appropriate cellular probes, has recently begun to be addressed, benefitting from the broader emerging area of chemoselective synthetic methodology. Thus, new reagents, catalysts and reaction processes are enabling both chemo- and site-selective modifications of complex, native natural products. In this review, we describe selected recent examples of these functionalization strategies in this emerging area.

 Please wait while we load your content...
Please wait while we load your content...