Solvothermal synthesis of MnFe2O4 colloidal nanocrystal assemblies and their magnetic and electrocatalytic properties†
Abstract
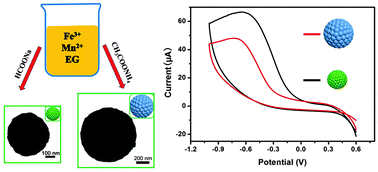
Submicrometer MnFe2O4 colloidal nanocrystal assemblies (CNAs) have been synthesized controllably by using a solvothermal method through simply adjusting synthetic reagents. The size and microstructure of MnFe2O4 CNAs were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Results showed that MnFe2O4 CNAs were well-separated and uniform with the size scales ranging from 230 nm to 950 nm, which were composed of primary crystalline nanoparticles with the sizes ranging from 16 nm to 43 nm. Room-temperature magnetic measurement results showed that MnFe2O4 CNAs were weakly ferromagnetic with small remnant saturation and coercivity values. The magnetization saturation values of CNAs were increased with the increase of the size of primary nanoparticles. Electrochemical measurements showed that the size of primary nanoparticles of MnFe2O4 CNAs had an important effect on the electrochemical reduction of H2O2. However, the electrocatalytic activity of MnFe2O4 CNAs for oxygen reduction reaction closely correlated with both the crystal size and self-assembly of primary nanoparticles. Based on the experimental results, the formation mechanisms of MnFe2O4 CNAs as well as the relationship between their structures and properties have been analyzed and discussed.

 Please wait while we load your content...
Please wait while we load your content...