Magnetic, electrochemical and optical properties of a sulfate-bridged Co(ii) imidazole dimer†
Abstract
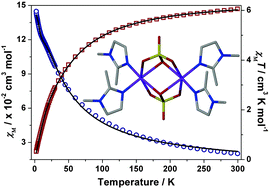
The synthesis, as well as the electrochemical, optical and magnetic properties of a sulfate-bridged Co(II) dimer, [Co2(DMIM)4(μ2-O2,O,O′-SO4)2]·2MeCN (DMIM = 1,2-dimethylimidazole) (2), are reported. The crystal structure consists of two distorted pseudo-octahedral Co(II) centres, each ligated by the μ2-(O-O′-sulfato) and μ2-(O2-sulfato) bridging modes of the SO42− anions. Magnetic studies have identified significant antiferromagnetic coupling between the high spin Co(II) centres, with J = −28 cm−1. Cyclic voltammetry indicated the presence of a quasi-reversible ligand centred oxidation, while the vis-NIR spectrum of 2 revealed complex splitting of the absorption bands due to the tetragonal distortion of the octahedral geometry of the Co(II) centres.

 Please wait while we load your content...
Please wait while we load your content...