Excellent fluoride removal properties of porous hollow MgO microspheres†
Abstract
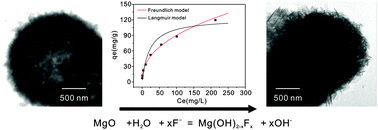
Porous hollow MgO microspheres were synthesized and characterized by X-ray diffraction, field emission scanning electron microscopy, transmission electron microscopy, and nitrogen adsorption–desorption isotherms. The removal properties of the MgO microspheres towards toxic fluoride were investigated, including adsorption kinetics, adsorption isotherms, and influences of pH and coexisting anions. The adsorption capacity was larger than 120 mg g−1 at a pH of 7.0. The effects of anions on fluoride removal were also investigated. The results indicated that phosphate was the greatest competitor of fluoride for adsorptive sites. In addition, the removal mechanism was revealed by Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy. The reaction of MgO and water molecules resulted in the formation of Mg(OH)2. The excellent adsorption properties towards fluoride resulted from the surface reaction between the generated Mg(OH)2 and fluoride in solution.

 Please wait while we load your content...
Please wait while we load your content...