Ni2+ ion assisted synthesis of hexagonal α-Fe2O3 nanoplates as anode materials for lithium-ion batteries†
Abstract
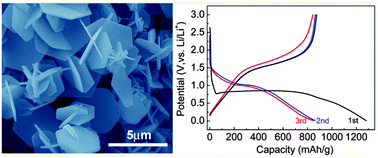
Fe2O3 hexagonal nanoplates have been successfully synthesized via a molten salt procedure with the assistance of Ni2+ ions. The resulting Fe2O3 is characterized by X-ray powder diffraction (XRD), field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). According to the microscopy observations, the morphology and size of the sample can be tailored by the foreign ions (Ni2+). Moreover, galvanostatic cell cycling is employed to evaluate the electrochemical performance of the obtained Fe2O3. The results show that the Fe2O3 hexagonal nanoplate electrodes have stable cycling performance with a reversible capacity of over 711 mA h g−1 after 100 cycles at a current density of 100 mA g−1. The sample with optimized structure shows the best rate performance with a high capacity of 418 mA h g−1 (40th cycle) even at a current density of 1000 mA g−1.

 Please wait while we load your content...
Please wait while we load your content...