Tuning the aggregation mode to induce different chiralities in organogels of mono- and bis-triterpenoid derivatives and the preparation of gold nanoparticles for use as a template†
Abstract
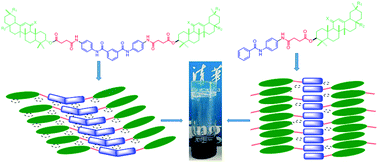
New gelators, based on chiral triterpenoids linked with aromatic rings and amide structures, were designed and synthesized. Different chiral properties of assembly of the organogels were observed and these were expressed by different close degrees of molecular packing, which were attributed to the formation of H-type aggregates in the monotriterpenoid and of J-type aggregates in the bis-triterpenoid derivatives. The nanofibers of the organogel in dimethylsulfoxide were used to engineer gold nanoparticles and generate gel–nanoparticle hybrid materials.

 Please wait while we load your content...
Please wait while we load your content...