Triazine–pyrimidine based molecular hybrids: synthesis, docking studies and evaluation of antimalarial activity†
Abstract
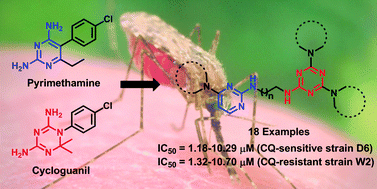
A series of novel triazine–pyrimidine hybrids have been synthesized and evaluated for their in vitro antimalarial activity. Some of the compounds showed promising antimalarial activity against both CQ-sensitive and CQ-resistant strains at micromolar level with a high selectivity index. All the compounds displayed better activity (IC50 = 1.32–10.70 μM) than the standard drug pyrimethamine (>19 μM) against the chloroquine-resistant strain W2. All the tested compounds were nontoxic against mammalian cell lines. Further, docking studies of the most active compounds were performed on both wild type and quadruple mutant (N51I, C59R, S108N, I164L) PfDHFR-TS using Glide to analyse the interaction of the compounds with the binding site of the protein. The binding poses of compounds 14 and 19, having a high Glide XP score and the lowest Glide energies, show an efficient binding pattern similar to that of the DHFR substrate (dihydrofolate) in the wild type and mutant DHFR active site. The analysis of the pharmacokinetic properties of the most active compounds using ADMET prediction attests to the possibility of developing compound 14 as a potent antimalarial lead.

 Please wait while we load your content...
Please wait while we load your content...