Homologous 1,3,5-triarylpyrazolines: synthesis, CH⋯π interactions guided self-assembly and effect of alkyloxy chain length on DNA binding properties†
Abstract
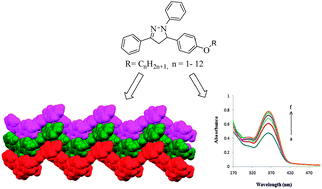
A series of new 2-pyrazoline derivatives (1c–12c) bearing one to twelve carbon homologous alkyloxy side chains have been synthesized in good to excellent yields via intramolecular cyclization reaction on treatment of chalcone intermediates (1a–12a) with phenyl hydrazine (1b) and characterized on the basis of their physical and spectral (IR, 1H & 13C NMR, GC-MS) data. The solid state structure of compound (2c) showed intriguing and unique 1D-supramolecular zigzag chain-like self-assembled structure, the driving force of which is only CH⋯π interactions. DNA interaction studies have also been carried out on selected compounds 1c, 3c, 5c, 6c, 9c and 12c of the series by UV-visible spectroscopy to evaluate their anticancer potential and the effect of alkyloxy chain length on DNA binding property. All the tested compounds showed strong DNA binding (105–106 M−1 binding constants) with hyperchromic effect. A slight increase in the DNA binding strength, observed on increasing the chain length of alkyloxy groups, was attributed to their conformational arrangements, leading to the best fit conformation of 1,3,5-triaryl moiety in the minor groove of DNA structure.

 Please wait while we load your content...
Please wait while we load your content...