Regulatory factors and the nature of Cu⋯Cu interaction in copper(i) complexes with NHC and NHCP ligands: a theoretical assessment
Abstract
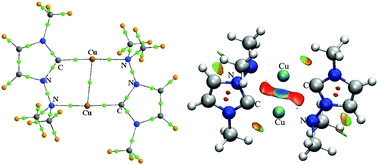
Factors affecting the Cu⋯Cu distance in copper(I) complexes with the N-heterocyclic carbene (NHC), bis-NHC and N-phosphinomethyl-functionalized NHC (NHCP) ligands have been investigated by quantum chemistry and topological analysis of electron density. The calculated results show that the ligands, ring size and shape, substitution pattern of NHC and NHCP, and overall charge of the system are factors that affect the Cu⋯Cu distance. The NHC ligand and an overall positive charge of the system lead to a short Cu⋯Cu distance. Topological analysis shows that there are attractions between the two Cu atoms. And the Cu⋯Cu attractions in the eight-membered systems and in the 10-membered cation systems are moderately strong and belong to the closed-shell type, which have partially covalent shared-closed interactions. Whereas the same interactions in the 12-membered cation system and in the neutral system with Br are weak and belong to closed-closed interactions.

 Please wait while we load your content...
Please wait while we load your content...