Efficient one-pot click synthesis of β-hydroxy-1,2,3-triazoles catalyzed by copper(i)@phosphorated SiO2via multicomponent reaction in aqueous media†
Abstract
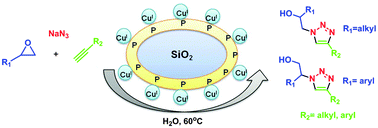
Copper(I)-modified SiO2, in particular Cu(I)@phosphorated SiO2 (CPSi) has been found to effectively catalyze the multi-component synthesis of β-hydroxy-1,2,3-triazoles from a variety of epoxides and alkynes in water. This novel heterogeneous catalyst is easily recycled and reused several times. The formation of the product proceeds in two reaction steps in one pot through a mechanism that involves in situ generated organic azide intermediate, followed by rapid ring closure of this intermediate with terminal alkynes to form β-hydroxy-1,2,3-triazole derivatives. The pure products were obtained in significantly high yields and short reaction times.

 Please wait while we load your content...
Please wait while we load your content...