Rapid synthesis of magnetite catalysts incorporated with M (Cu, Ni, Zn, and Co) promoters for high temperature water gas shift reaction
Abstract
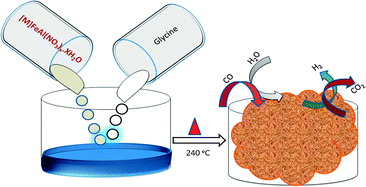
A rapid one step direct synthesis of high temperature water gas shift (HT-WGS) catalysts, magnetite incorporated with various promoters like Cu, Ni, Zn, and Co, has been shown. The key achievements in this work, in terms of catalyst preparation, are as follows: (1) direct synthesis of magnetite, a catalytically active phase in HT-WGS reaction, (2) easy incorporation of various transition metal promoters like Cu, Ni, Zn, and Co, (3) rapid preparation without any washing step by using an ample amount of solvent, and (4) a simplified procedure by avoiding longer digestion time and any specific pH value. To achieve this, an amino acid, glycine, was used as a complexing agent as well as a fuel for the auto-combustion process. The zwitterionic nature of glycine favors the easy incorporation of the promoter metal ions. In addition, the reducing atmosphere created by glycine during its burning makes it possible to prepare magnetite directly. During the HT-WGS reaction, the activity follows the order Cu-FAG > Ni-FAG > Zn-FAG > Co-FAG. The optimization of the Cu promoter is also carried out. At 12.5 mol% of Cu loading the catalytic activity reached a CO conversion value of 87% at 400 °C.

 Please wait while we load your content...
Please wait while we load your content...