Synthesis and study of (R)- and (S)-β-hydroxyphosphonate acyclonucleosides as structural analogues of (S)-HPMPC (cidofovir)†
Abstract
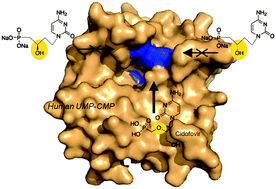
A synthetic pathway to new acyclonucleoside phosphonates, designed as analogues of cidofovir, is described. The reduction of a β-ketophosphonate intermediate, readily available from the nucleobase and benzylacrylate, afforded an enantiomeric mixture of (R)- and (S)-β-hydroxyphosphonate derivatives which was resolved. The assignment of the absolute configuration was proposed on the basis of NMR studies. The influence of this modification, the presence of the hydroxyl group and chirality on the β-position related to the phosphorus atom, on antiviral activity against a broad variety of DNA and RNA viruses and also on the capacity to be recognized as substrates by human NMP kinases was investigated.

 Please wait while we load your content...
Please wait while we load your content...