New aminotetrazole derivatives as hydrogen bonding catalysts. A green and selective oxidation of organosulphides with H2O2 in H2O†
Abstract
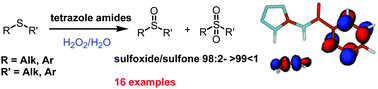
The oxidation of organosulphides catalysed by hydrogen bonding donors derived from aminotetrazole has been studied. The oxidation reaction was performed in a H2O solution using H2O2 as a versatile, green and chemoselective new approach to sulphoxides. Sulphoxide compounds are obtained in high yields and excellent selectivity through a new and easy to perform oxidation protocol. Aminotetrazole derivatives can be recycled by filtration and reused several times without expensive purification procedures.

 Please wait while we load your content...
Please wait while we load your content...