New highly electrodeficient cationic fluorescent tetrazines: a step toward the strongest purely organic photooxidants†
Abstract
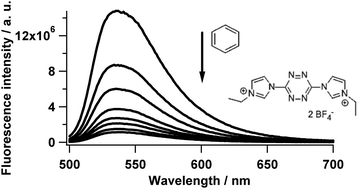
Strongly fluorescent electrodeficient tetrazines substituted with cationic heterocycles have been prepared. These compounds can be reversibly reduced at a high potential and consequently they are strong oxidants in the excited state. As a result of their very strong photooxidant character, their fluorescence is quenched in the presence of toluene, m-xylene, styrene and sometimes even benzene, through a photoinduced electron transfer reaction via a dynamic quenching mechanism. Beyond their photooxidizing properties, these new molecules have potential towards the realization of new fluorescence sensors. As an example, tetrazine 3 has been dispersed on silica particles and we have demonstrated that its fluorescence is quenched upon exposure to benzene vapors.

 Please wait while we load your content...
Please wait while we load your content...