Helix foldamers of γ-peptides based on 2-aminocyclohexylacetic acid: a computational study†
Abstract
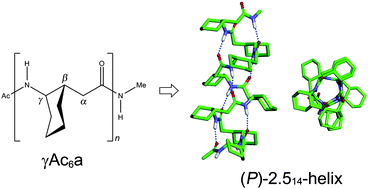
The conformational preferences of helix foldamers have been studied for oligo-γ-peptides of 2-aminocyclohexylacetic acid (γAc6a) with a cyclohexyl constraint on the Cβ–Cγ bond using density functional methods. For the di-γAc6a peptide with the homochiral (1S,2S) configurations, the right-handed (P) 9-helical structure is found to be the most preferred in the gas phase, whereas the (P) 14-helical structure is favored by ∼0.2 kcal mol−1 over the former in water. As the peptide sequence becomes longer, the (P)-2.514-helices are the most preferred for tetra- to octa-γAc6a peptides both in the gas phase and in water, which is due to the favored conformational energies. The calculated mean backbone torsion angles and helical parameters of the 14-helix foldamers of oligo-γAc6a peptides are consistent with those of the X-ray structures of Cα-ethyl-γAc6a peptides. Although the 14-helix foldamers of γAc6a and 2-(aminomethyl)cyclohexanecarboxylic acid (γAmc6) oligopeptides have similar mean backbone torsion angles and helical parameters, there are some differences in structure and/or helix handedness for other helix foldamers, which can be ascribed to the different substitutions of the cyclohexyl constraint on Cβ–Cγ or Cα–Cβ bonds. Therefore, the conformational preferences of the oligo-γAc6a peptides obtained here are expected to provide useful information for structure-based designs of biologically active γ-peptides with specific functions.

 Please wait while we load your content...
Please wait while we load your content...