Vacuolar-type H+-ATPase subunits and the neurogenic protein big brain are required for optimal copper and zinc uptake†
Abstract
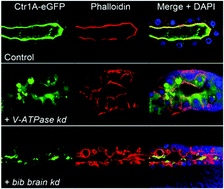
Copper and zinc homeostasis in polarized epithelial cells requires the correct localization and regulation of membrane-bound transport proteins at the apical and basolateral cell membranes. We have identified a subunit of the vacuolar-type H+-ATPase (V-ATPase) complex, vhaPPA1-2, and the Drosophila aquaporin homolog big brain (bib), as being required for the correct localization of the copper uptake transporters Ctr1A and Ctr1B and the zinc uptake protein dZip89B and hence necessary for optimal copper and zinc accumulation in vivo. Knockdown of vhaPPA1-2 or bib resulted in cuticle hypo-pigmentation phenotypes typical of copper deficiency in the fly and induction of midgut Ctr1B expression, a known response to low cellular copper levels. Furthermore, midgut-specific knockdown of bib increased tolerance to elevated dietary zinc levels. Ctr1A, Ctr1B and dZip89B are normally localized to the apical plasma membrane. Upon knockdown of vhaPPA1-2 or bib, this localization was strongly disrupted as was that of the generic plasma membrane marker CD8-GFP, indicating that these two genes are not acting specifically on metal ion homeostasis but rather are necessary for general apical membrane protein localization in polarized epithelial cells. These results suggest that metal ion transport is particularly sensitive to disturbances in cellular protein localization processes.

 Please wait while we load your content...
Please wait while we load your content...