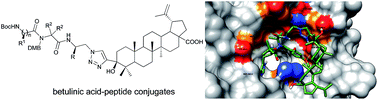
Synthesis of new betulinic acid–peptide conjugates and in vivo and in silico studies of the influence of peptide moieties on the triterpenoid core activity
Abstract
The modification of betulinic acid derivatives bearing an ethynyl group at the C-3 position by different azidopeptides using Cu(I)-catalyzed

 Please wait while we load your content...
Please wait while we load your content...