More than just a GPCR ligand: structure-based discovery of thioridazine derivatives as Pim-1 kinase inhibitors†‡
Abstract
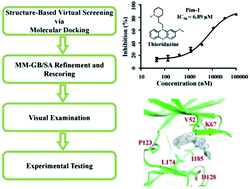
Pim-1 kinase is a serine/threonine kinase which plays an important role in cell proliferation and differentiation. The Pim-1 kinase expression is elevated in leukemia and prostate cancer. Accordingly, we employed a structure-based hierarchical virtual screening approach to identify potential unknown Pim-1 kinase activity for existing drugs. Among the LOPAC library of pharmacologically active compounds, one top-ranked drug molecule thioridazine, a well-known antipsychotic agent which exerted its biological function as a dopamine receptor antagonist, showed low micromolar activity in the Pim-1 enzymatic assay. We determined the co-crystal structure of thioridazine bound with Pim-1 kinase, and defined the key elements of the pharmacophore by analyzing the structure–activity relationship of thioridazine analogues. In addition, we also assessed our pharmacophore by successfully predicting the Pim-1 activity of the selective Akt inhibitor, 10-DEBC. Our discovery of the unknown Pim-1 inhibitory activity of thioridazine and 10-DEBC might provide novel insights into understanding their molecular mechanism of action, and inspire the computation-driven multiple-target drug discovery.

 Please wait while we load your content...
Please wait while we load your content...