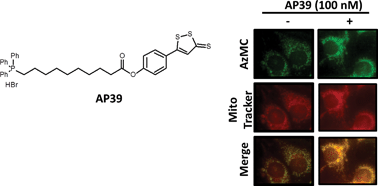
The synthesis and functional evaluation of a mitochondria-targeted hydrogen sulfide donor, (10-oxo-10-(4-(3-thioxo-3H-1,2-dithiol-5-yl)phenoxy)decyl)triphenylphosphonium bromide (AP39)
Abstract
Synthesis and bioavailability of the endogenous gasomediator hydrogen sulfide (H2S) is perturbed in many disease states, including those involving mitochondrial dysfunction. There is intense interest in developing pharmacological agents to generate H2S. We have synthesised a novel H2S donor molecule coupled to a mitochondria-targeting moiety (triphenylphosphonium; TPP+) and compared the effectiveness of the compound against a standard non-TPP+ containing H2S donor (GYY4137) in the inhibition of oxidative stress-induced endothelial cell death. Our study suggests mitochondria-targeted H2S donors are useful pharmacological tools to study the mitochondrial physiology of H2S in health and disease.

 Please wait while we load your content...
Please wait while we load your content...