Molecular docking and molecular dynamics studies on the structure–activity relationship of fluoroquinolone for the HERG channel
Abstract
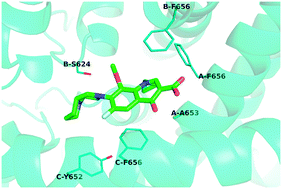
Fluoroquinolones play an important role in the treatment of serious bacterial infections, but at the same time they could lead to cardiac toxicity due to the blockage of the HERG potassium channel, which even leads to the withdrawal of some fluoroquinolones. Blockage of the HERG potassium channel by drugs or drug-like compounds has become a critical problem in drug discovery. Though there were large amounts of bioactivity data of fluoroquinolones on the blockage of HERG, little structural basis of binding of blockers to the HERG channel was known. Here, we combined molecular docking, molecular dynamics simulations, free energy calculations and binding energy decomposition analysis to explore the binding modes of fluoroquinolones in the HERG potassium channel. The calculated binding free energies were consistent with the experimental binding affinities. Our results showed that the CH3 group in MX was favorable for the binding to the HERG channel, while Tyr652 and Phe656 were critical for the hydrophobic interaction between fluoroquinolones and the HERG channel. We expected that our results of calculation could provide important insights for the rational design and discovery of drugs.

 Please wait while we load your content...
Please wait while we load your content...