Continuous flow real-time PCR device using multi-channel fluorescence excitation and detection
Abstract
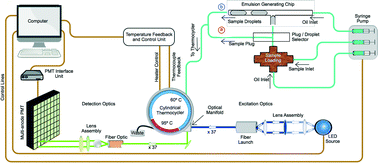
High throughput automation is greatly enhanced using techniques that employ conveyor belt strategies with un-interrupted streams of flow. We have developed a ‘conveyor belt’ analog for high throughput real-time quantitative Polymerase Chain Reaction (qPCR) using droplet emulsion technology. We developed a low power, portable device that employs LED and fiber optic fluorescence excitation in conjunction with a continuous flow thermal cycler to achieve multi-channel fluorescence detection for real-time fluorescence measurements. Continuously streaming fluid plugs or droplets pass through tubing wrapped around a two-temperature zone thermal block with each wrap of tubing fluorescently coupled to a 64-channel multi-anode PMT. This work demonstrates real-time qPCR of 0.1–10 μL droplets or fluid plugs over a range of 7 orders of magnitude concentration from 1 × 101 to 1 × 107. The real-time qPCR analysis allows dynamic range quantification as high as 1 × 107 copies per 10 μL reaction, with PCR efficiencies within the range of 90–110% based on serial dilution assays and a limit of detection of 10 copies per rxn. The combined functionality of continuous flow, low power thermal cycling, high throughput sample processing, and real-time qPCR improves the rates at which biological or environmental samples can be continuously sampled and analyzed.
- This article is part of the themed collection: Microtechnologies in Medicine and Biology (MMB 2013)

 Please wait while we load your content...
Please wait while we load your content...