Transition-metal free 2-arylbenzoxazole formation from aryl amides and cyclohexanones†
Abstract
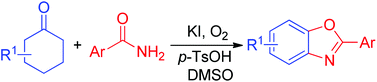
A transition-metal-free method for the synthesis of 2-arylbenzoxazoles from readily available cyclohexanones and benzamides is described. The combined use of KI, p-TsOH and DMSO significantly improved the reaction yields. Non-aromatic cyclohexanones were smoothly dehydrogenated and acted as the aryl source using oxygen as the oxidant.

 Please wait while we load your content...
Please wait while we load your content...