Efficient 4,5-dihydro-1H-imidazol-5-one formation from amidines and ketones under transition-metal free conditions†
Abstract
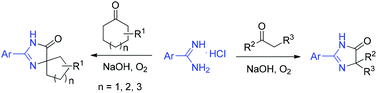
An efficient procedure for 4,5-dihydro-1H-imidazol-5-one preparation from aryl amidines and ketones under transition-metal free conditions is described. When cyclic ketones were employed, various spiro-fused 4,5-dihydro-1H-imidazol-5-ones were formed in high yields via rearrangement reaction.

 Please wait while we load your content...
Please wait while we load your content...