The carbon material functionalized with NH2+ and SO3H groups catalyzed esterification with high activity and selectivity†
Abstract
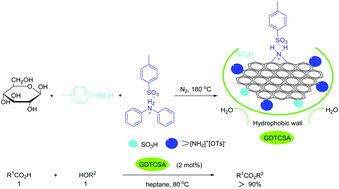
A novel carbon-based solid acid was conveniently prepared by heating a mixture of D-glucose, p-toluenesulfonic acid and diphenylammonium tosylate. Its structure was measured by XRD, FT-IR, XPS, 13C MAS NMR and EA to illustrate that the carbon material has been functionalized with NH2+ and SO3H groups and has a strong “hydrophobic effect”. It can be used to catalyze the esterification reaction of carboxylic acids with equimolar amounts of sterically demanding and acid-sensitive alcohols with high reactivity (yield up to 90%) and selectivity (up to 95%) in heptane at 80 °C. It could be easily recovered and reused more than ten times without loss of activity.

 Please wait while we load your content...
Please wait while we load your content...