The relationship between oxidation and hydrolysis in the conversion of cellulose in NaVO3–H2SO4 aqueous solution with O2†
Abstract
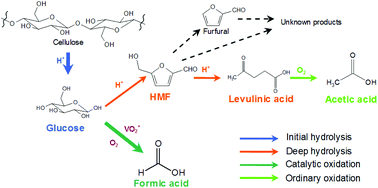
Conversion of cellulose, the most abundant biomass, shows a high selectivity to yield formic acid (FA), when oxidized by O2 in NaVO3–H2SO4 aqueous solution. This conversion involves various reactions including hydrolysis and oxidation. In this work, the relationships between these reactions were studied. There are mainly two hydrolyses and two oxidations occurring in the conversion: initial hydrolysis (from cellulose to monosaccharides) and deep hydrolysis (from monosaccharides to levulinic acid); catalytic oxidation (from monosaccharides to FA) and ordinary oxidation (from levulinic acid to acetic acid). Among these four reactions, catalytic oxidation to FA and deep hydrolysis to byproducts are competitive. The increasing rate of deep hydrolysis is faster than that of catalytic oxidation, when temperature is increased. Catalytic oxidation is promoted and deep hydrolysis is suppressed, when the O2 pressure is increased. Moreover, deep hydrolysis is promoted and catalytic oxidation is suppressed, when H2SO4 concentration is increased. The hydrolysis–oxidation pathway of this conversion was proposed. Thus, the byproducts could be inhibited, and FA-oriented transformation could be realized.

 Please wait while we load your content...
Please wait while we load your content...