Catalytic transesterification of cellulose in ionic liquids: sustainable access to cellulose esters
Abstract
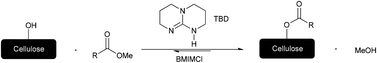
Catalytic transesterifications of cellulose were studied under homogeneous conditions using the ionic liquid 1-butyl-3-methylimidazolium chloride (BMIMCl) as a solvent. Cellulose was thus efficiently converted into cellulose esters employing various methyl esters and 10 mol% of 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) as catalyst. 1H NMR analysis of the products revealed up to 2.3 turnovers of the methyl esters per catalyst molecule, leading to degrees of substitution (DS) of up to 0.69. Although a comparatively low turnover number (TON) is observed, the developed methodology represents the first successful homogeneous catalytic reaction on cellulose. Furthermore, the new method is an important step forward in terms of sustainability, since the BMIMCl–DMSO mixture can be recycled and reused for the reaction, and toxic and corrosive chemicals commonly employed for cellulose esterification (such as anhydrides, acid chlorides and bromides, organic bases, all in overstoichiometric amounts) are avoided. To demonstrate the versatility of this transesterification, an aromatic (cellulose benzoate), an aliphatic (cellulose butyrate), and a fatty acid containing cellulose ester (cellulose 10-undecenoate) were prepared. Additionally, cellulose 10-undecenoate was successfully used for thiol–ene grafting onto reactions employing two thiols for efficient thiol–ene addition reactions.

 Please wait while we load your content...
Please wait while we load your content...