Pd-catalyzed ethylene methoxycarbonylation with Brønsted acid ionic liquids as promoter and phase-separable reaction media†
Abstract
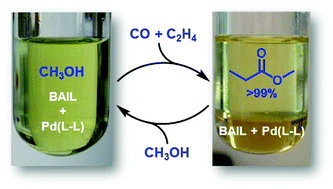
Brønsted acid ionic liquids (BAILs) were prepared and applied as combined acid promoters and reaction media in Pd–phosphine catalyzed methoxycarbonylation of ethylene to produce methyl propionate. The BAILs served as alternatives to common mineral acids required for the reaction, e.g. methanesulfonic acid or sulfuric acid, resulting in high catalytic activity and selectivity towards methyl propionate. In addition, the BAILs yielded a biphasic system with the product and provided stability to palladium intermediates avoiding the undesirable formation of palladium black after reaction. These special features enabled facile methyl propionate separation and recovery of the ionic liquid catalyst system, thus allowing its re-use up to 15 times without apparent loss of catalytic activity or selectivity.
- This article is part of the themed collection: 5th Congress on Ionic Liquids

 Please wait while we load your content...
Please wait while we load your content...