The importance of charge-transfer interactions in determining chromophoric dissolved organic matter (CDOM) optical and photochemical properties
Abstract
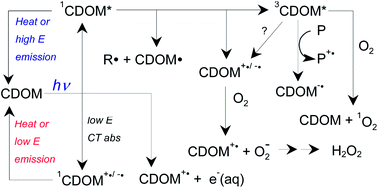
Absorption of sunlight by chromophoric dissolved natural organic matter (CDOM) is environmentally significant because it controls photic zone depth and causes photochemistry that affects elemental cycling and contaminant fate. Both the optics (absorbance and fluorescence) and photochemistry of CDOM display unusual properties that cannot easily be ascribed to a superposition of individual chromophores. These include (i) broad, unstructured absorbance that decreases monotonically well into the visible and near IR, (ii) fluorescence emission spectra that all fall into a single envelope regardless of the excitation wavelength, and (iii) photobleaching and photochemical quantum yields that decrease monotonically with increasing wavelength. In contrast to a simple superposition model, these phenomena and others can be reasonably well explained by a physical model in which charge-transfer interactions between electron donating and accepting chromophores within the CDOM control the optical and photophysical properties. This review summarizes current understanding of the processes underlying CDOM photophysics and photochemistry as well as their physical basis.
- This article is part of the themed collection: Aquatic photochemistry

 Please wait while we load your content...
Please wait while we load your content...