Molecular catalysts for hydrogen production from alcohols
Abstract
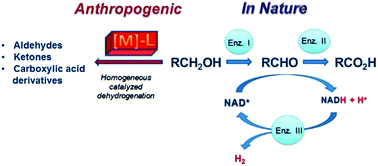
An industrially applicable catalytic methodology for dihydrogen formation from a proton source remains at the forefront of all efforts to replace the present fossil fuel economy by a hydrogen economy. This review tries to summarize the achievements which have been made with molecular organometallic complexes as catalysts for the dehydrogenation of alcohols. Biology uses NAD+ as a metal-free hydrogen acceptor which converts with the help of enzymes (alcohol dehydrogenase, aldehyde dehydrogenase) alcohols in carbonyl compounds, NADH, and protons. In the regeneration of NADH to NAD+, electrons are stored in electron transfer enzymes (ferredoxines) which are subsequently used to reduce protons to hydrogen with the help of hydrogenases or nitrogenases which ensures a very low overpotential for the reduction. Man-made organometallic complexes are rather primitive with respect to this complex machinery but use some principles from biology as guide lines. Classical complexes like rhodium or ruthenium phosphane complexes achieve at best a few thousands of turn over frequencies (TOFs). Established reactions like oxidative addition of the hydroxyl group of the substrate to the metal centre, β-hydrogen elimination from the α-CH group of the coordinated alcohol, product dissociation, and reductive elimination of hydrogen are involved in the proposed catalytic cycles. Complexes which show metal–ligand cooperativity show a significantly better performance with respect to turn over frequencies (conversion rate = activity) and turn over numbers (number of product molecules per catalyst molecule = efficiency). In these catalytic systems, the alcohol substrate is converted with the help of active centres in the ligand backbone which participate directly and reversibly in the transformation of the substrate. Present results indicate that dehydrogenative coupling reactions of the type, R–CH2–OH + XH → RCOX + 2H2, proceed especially well and can be applied to a wide range of substrates including multiple dehydrogenative couplings leading to polyesters or polyamides. In photocatalytic conversions, alcohols can be deoxygenated to hydrocarbons, CO, and H2 which should be further explored in the future. New developments consist of the construction of organometallic fuel cells (OMFCs) where the anode is composed of molecular catalysts embedded into a conducting support material. Here no free hydrogen is evolved but it is directly converted to electric current and protons according to H2 → 2H+ + 2e. The review focuses on the catalysis with organometallic complexes but lists some selected results obtained with heterogeneous catalytic systems for comparison.

 Please wait while we load your content...
Please wait while we load your content...