FeFe hydrogenase reductive inactivation and implication for catalysis†
Abstract
We show that FeFe hydrogenases inactivate at low potential, in a complex process that is mostly reversible. A form of the enzyme that is produced slowly and reversibly under reductive conditions has no proton activity under reductive conditions, although it can still oxidize H2 under oxidative conditions. This suggests that the so-called “super-reduced” state of the active site H-cluster is not part of the normal catalytic cycle. We also discuss our findings in relation to the optimization of H2-photoproduction devices based on FeFe hydrogenases that receive electrons from low potential photosensitizers.
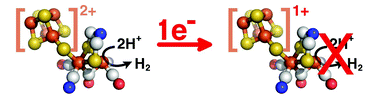

 Please wait while we load your content...
Please wait while we load your content...