Noncovalent interactions determine the conformation of aurophilic complexes with 2-mercapto-4-methyl-5-thiazoleacetic acid ligands†
Abstract
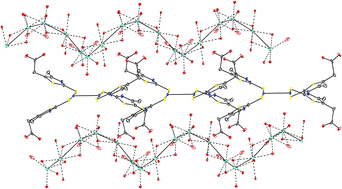
We report the synthesis of three gold(I) complexes [Au(H2-mmta)2]Cl·3H2O (1), Na3[Au(mmta)2]·6H2O (2) and Na3[Au(mmta)2]·10.5H2O (3) (H2-mmta = 2-mercapto-4-methyl-5-thiazoleacetic acid) in which the Au(I) centre is incorporated either in cationic or anionic units of the [Au(SR)2]+/− type depending on the protonation state of the ligand. All structures were characterized by single crystal X-ray analysis and found to exhibit unsupported aurophilic interactions leading to the formation of dimeric [Au2(H2-mmta)4]2+ and [Au2(mmta)4]6− species. By applying several ab initio interpretative techniques we examine the character of the intermolecular interactions stabilizing the eclipsed arrangement of the aurophilic dimers formed in 1–3.

 Please wait while we load your content...
Please wait while we load your content...