Sustainable synthetic route for γ-Fe2O3/C hybrid as anode material for lithium-ion batteries†
Abstract
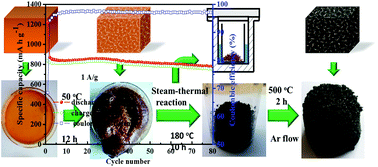
A facile, high-yield and sustainable method is developed to synthesize iron oxide/C hybrids. Starch is chosen as the carbon source due to its superior gelatinization property and natural abundance, and ferric nitrate is used as the iron salt for the sustainable synthesis. The iron oxide in the final products exists in the γ-Fe2O3 phase. The γ-Fe2O3/C hybrids are used as anode materials for lithium-ion batteries. The batteries exhibit better cyclability as the content of γ-Fe2O3 decreases, but in turn the reversible capacity declines. The γ-Fe2O3/C hybrid with 63.96 wt% of γ-Fe2O3 has an initial discharge capacity of 1149 mA h g−1 and after the 80th cycle the reversible capacity is maintained at over 720 mA h g−1 at a current density of 0.5 A g−1. Even when tested at a current density of 5 A g−1, a substantial discharge capacity of ∼300 mA h g−1 can be obtained.

 Please wait while we load your content...
Please wait while we load your content...