A series of cobalt and nickel clusters based on thiol-containing ligands accompanied by in situ ligand formation†
Abstract
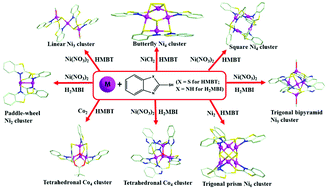
Eight cobalt and nickel clusters with the formulae [Co4(μ4-O)(MBT)5(μ2-Piv)] (1), [Ni3(MBT)2(L1)2(OCH3)2] (2), [Ni4(μ3-S)(μ3-S2)(MBT)2(L2)] (3), [Ni4(L3)4] (4), [Ni6(μ4-S)3(MBT)6]·(C2H5OH)2 (5), H[Co4(μ4-O)(HMBI)6]·(NO3)(TEA)0.5(CH3OH)2(H2O) (6), [Ni2(HMBI)4]·(CH3OH)2 (7), and [Ni5(MBI)2(HMBI)4(OCH3)2]·(CH3OH)3(H2O)2 (8) (HPiv = pivalic acid, HL1 = 2-disulfanylbenzo[d]thiazole, H2L2 = (Z)-2-((2-mercaptophenyl)imino)benzo[d]thiazole-3(2H)-thiol, H2L3 = (Z)-2-(benzo[d]thiazol-2(3H)-ylideneamino)benzenethiol, TEA = triethylamine) have been solvothermally prepared via assembling distinct metal resource and thiol-containing ligands 2-mercaptobenzothiazole (HMBT)/2-mercaptobenzimidazole (H2MBI). Complexes 1 and 6 are tetrahedral cobalt clusters. Complex 2 features linear arrangement of nickel ions. Complexes 3 and 4 are tetranuclear nickel clusters with the butterfly and square shape, respectively. Complex 5 displays a trigonal prism geometry. Complexes 7 and 8 exhibit paddle-wheel and trigonal bipyramidal geometry, respectively. The starting ligand HMBT undergoes in situ ligand transformation in the formation of the nickel clusters, and the new generated inorganic ligands (S2− and S22−) and organic ligands (HL1, H2L2 and H2L3) were captured within the metallic cores. Magnetic studies indicate that complexes 1 and 6 show dominating antiferromagnetic couplings and that spin frustration exists in 6.

 Please wait while we load your content...
Please wait while we load your content...