An acid-stable Zn(ii) complex: electrodeposition in sulfuric acid and the effect on the zinc–lead dioxide battery†
Abstract
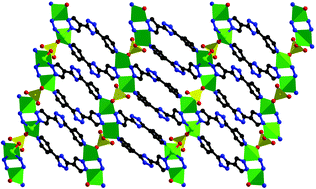
An acid-stable Zn(II) complex formulated as Zn2(HL)2(SO4)·H2O (1) and an acid-unstable complex formulated as Zn2L2·12H2O (2) were hydro(solvo)thermally synthesized and structurally characterized by single-crystal X-ray diffraction. Complex 1 features a uninodal 6-connected 2-fold interpenetrating three-dimensional (3D) dense architecture with {412·63}-pcu topology, and complex 2 exhibits a 2-nodal (3, 6)-connected 3D open architecture with (4·62)2(42·610·83)-rtl topology. The results indicate that the stability of complex 1 in sulfuric acid is probably associated with the coordinated SO42− in the quite dense structure, and complex 1 can also be synthesized via electrodeposition in sulfuric acid; it can improve the discharging characteristics of the zinc–lead dioxide battery at room temperature.

 Please wait while we load your content...
Please wait while we load your content...