A Co(ii) thiocyanato coordination polymer with 4-(3-phenylpropyl)pyridine: the influence of the co-ligand on the magnetic properties†‡
Abstract
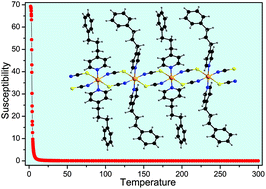
Three new coordination compounds with the composition Co(NCS)2(4-(3-phenylpropyl)pyridine)4 (1), Co(NCS)2(4-(3-phenylpropyl)pyridine)4(H2O)2 (2) and [Co(NCS)2(4-(3-phenylpropyl)pyridine)2]n (3) were prepared and investigated. The crystal structures of compounds 1 and 2 consist of discrete complexes, in which the Co(II) cations are coordinated by only terminal N-bonded thiocyanato anions. In the crystal structure of 3 the Co(II) cations are linked into chains by pairs of μ-1,3-bridging thiocyanato anions. DTA-TG measurements on compound 1 show decomposition without the formation of 3 as an intermediate. In contrast, on heating compound 2 two water molecules are removed in the first step leading to the formation of compound 3 in the second step. Magnetic measurements on 3 reveal ferromagnetic interactions between Co(II) ions along chains with J = 29.5(1) K, and also ferromagnetic interactions between chains with zJ′ = 0.38(1) K. The ferromagnetic transition is observed at 3.3 K, which is confirmed by specific heat measurements. The temperature dependent ac susceptibility shows slow relaxations above and below 3.3 K. The results for this quasi-one dimensional Ising ferromagnet, having also some features of a cluster spin-glass, are compared with those of related compounds.

 Please wait while we load your content...
Please wait while we load your content...