The unusual binding mechanism of Cu(ii) ions to the poly-histidyl domain of a peptide found in the venom of an African viper†
Abstract
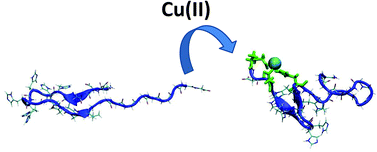
Copper complexes of a poly-His/poly-Gly peptide (EDDHHHHHHHHHGVGGGGGGGGGG-NH2), a natural component of a snake venom, were studied by means of both experimental (thermodynamic, spectroscopic and MS) techniques and molecular dynamics (MD) simulations and density functional theory (DFT) calculations. This peptide proved to be an exceptionally effective copper chelator, forming complexes which are thermodynamically more stable than those formed by both the albumin-like ATCUN motif and several other poly-histidine protein fragments. We show that, in a poly-histidine stretch, copper seems to prefer binding to residues separated by one amino acid and that a correlation between an α-helical structure of the predicted complexes and their thermodynamic stability is observed.

 Please wait while we load your content...
Please wait while we load your content...