Mononuclear complexes of amide-based ligands containing appended functional groups: role of secondary coordination spheres on catalysis†
Abstract
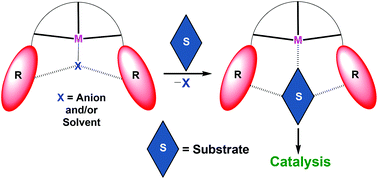
Amide-based ligands H2L1, H2L2 and H2L3 containing thiazole, thiazoline and benzothiazole appended groups have been used to synthesize Zn2+ (1 and 3), Cd2+ complexes (2 and 4), and a Mn2+ complex (5). In all cases, potentially multidentate ligands create a meridional N3 coordination environment around the M(II) ion whereas additional sites are occupied by labile nitrate ions in 1–4 and MeOH in 5. Interestingly, metal complexation caused the migration of protons from amidic N–H sites to the appended heterocyclic rings in complexes 1–4. Structural studies show that the protonated heterocyclic rings in these complexes create a hydrogen bond based cavity adjacent to the metal ion. Importantly, binding studies confirm that the substrates are bound within the complex cavity closer to the Lewis acidic metal in all complexes including the oxidation-sensitive Mn ion in complex 5. All complexes have been utilized as the reusable and heterogeneous catalysts for ring-opening reactions of assorted epoxides, cyanation reactions of various aldehydes, and epoxidation reactions of several olefins.

 Please wait while we load your content...
Please wait while we load your content...